20 YEARS RESEARCH STUDIES NOT ALL ZEOLITES ARE ALIKE!!
PMA means safety, homogeneity, usability and effectiveness of the products.
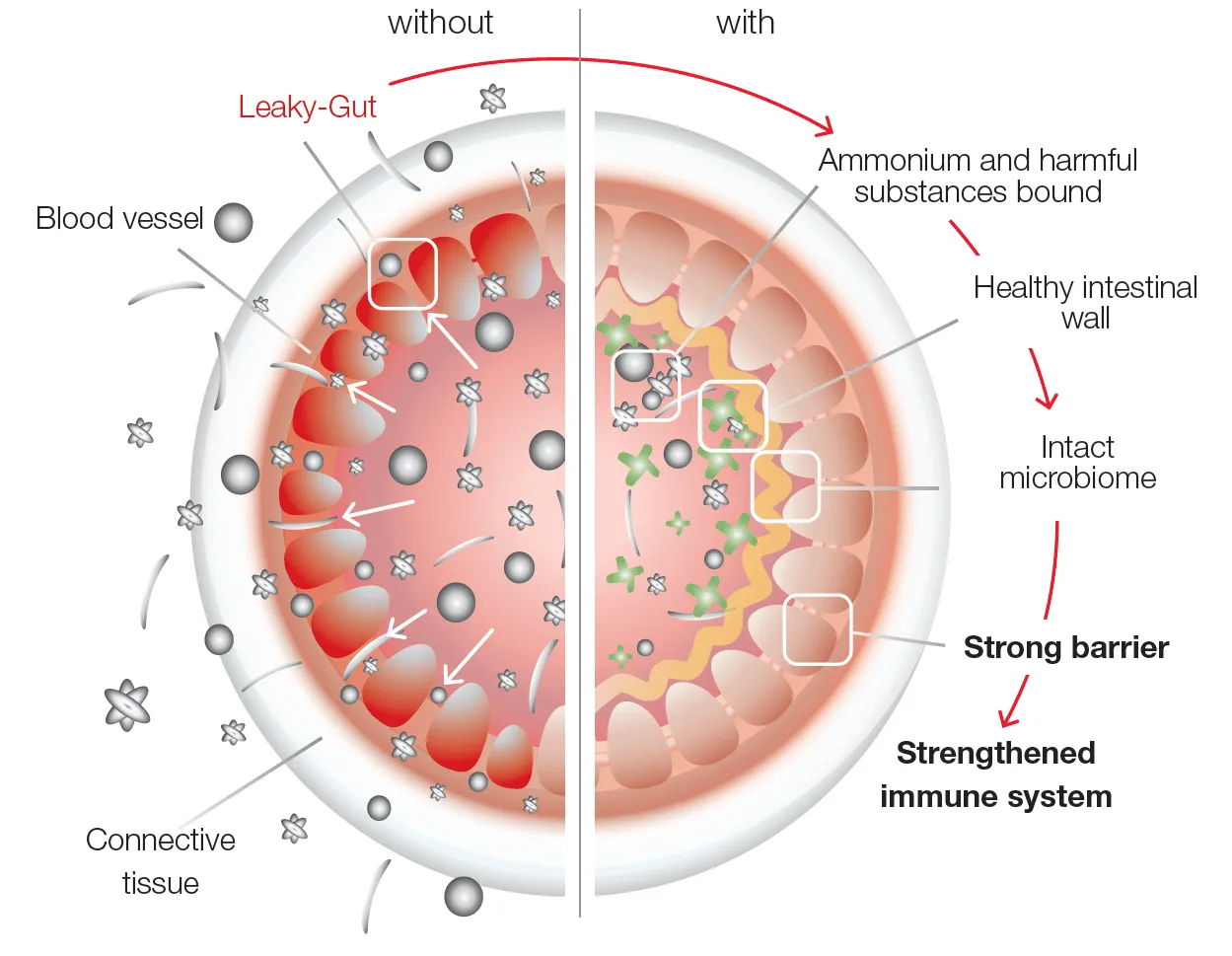
Strength and Power with your Healthy Intestine
Diseases which are associated with an increased permeability of the intestinal wall:
- Irritable bowel (nervous or inflammatory)
- Chronic inflammatory bowel disease (IBD): Crohn's disease, collitis ulcerosa
- Elevated liver values or non-alcoholic fatty liver
- Food intolerances/allergies
- Autoimmune reactions: Rheumatoid arthritis, celiac disease and multiple sclerosis, diabetes type1 and type2
- Chronic Fatigue Syndrome
- Depression
- Skin - neurodermatitis, psoriasis, acne
Symptoms:
Abdominal cutting
abdominal cramps
flatulence
bloating
hard stool <-> diarrhoea, sleeping problems
Fatigue
Allergies, autoimmune diseases Susceptibility to infections ....
... depression
How can PMA zeolite help?
- Neutralise stressors - bind pollutants
- Influence the intestinal environment favourably (sustainable additive effect to probiotics)
- Protect „good“ (useful) gut bacteria
- Restore the barrier of the intestinal wall
- Support the immune system (barrier function)
- Reduce local & systemic inflammation
- Relieve the burden on "downstream" systems/organs
- Preventing or alleviating secondary diseases
PMA ZEOLITE THE ORIGINAL
In the first phase, it was of crucial importance to demonstrate the safety of the active ingredient. Very early on, exceptional positive efficacy on oxidative stress, significant lactate reduction during physical exertion, a substantial contribution to detoxification and exceptional positive efficacy on the intestinal wall barrier emerged. These effects were accompanied by the selective binding and elimination of very specific toxins and ammonium in the gastrointestinal tract. Another important step was the certification as a medical device in 2006, in order to meet the extremely high and strict quality standards required by law for this natural product.
THE EUROPEAN GENERAL MEDICAL DEVICE NOMENCLATURE DEFINED ZEOLITE AS FOLLOWS
Mineral-based gastrointestinal detoxifier
An orally-administered substance principally comprised of a mineral such as zeolite, intended to absorb, adsorb, and/or chelate and remove harmful exogenous and/or autologous toxins/substances (for example, heavy metals, ammonium, microbial toxins, pesticides, histamine, serotonin, alcohol, and/or bile acids) from some or most of the gastrointestinal (GI) tract. It may also function as an antioxidant. It is provided in various forms (for example, powder, capsule, ) and is normally available non-prescription over-the-counter (OTC) for use in the home or health care facility. This is a single-use device.

WHAT MEANS PMA ZEOLITE?
PMA-zeolite® is obtained by double activation, an exclusive patented technology that reduces the surface area per unit weight. PMA-zeolite achieves 4000 square meters per gram. It is the only zeolite to have performed a complete toxicity study, acute, sub-chronic and chronic, and these studies only apply to this molecule as the activated molecule is very different from the starting mineral.
PMA-zeolite® is perhaps, in the whole history of 'detoxifiers and detoxicants', the most complex, complete and effective intervention system when compared to the current man-made environment.
Why an activated zeolite when nature has already given us natural zeolite? Imagine a porous sponge composed of millions of channels, capable of collecting dirt; how to reach the inner ones? Simple: by tearing it apart. In PMA technology as soon as the zeolite particles enter the chamber, they move towards the central axis of the air vortices, each rotating in one of two directions and impacting at supersonic speed against those coming from the opposite direction. In doing so, the irregularity of the particles results in very small parts, each developing a maximum surface area. Here our sponge has reached its maximum efficiency. But interaction with nature leads to sometimes unexpected discoveries: zeolite treated in this way turns out to have an increased surface charge. Here is the activation value: PMA-zeolite® see the short film
To discriminate between the different technologies, their names and applications, see bibliography:
Zeolite Clinoptilolite: Therapeutic Virtues of an Ancient Mineral
SUMMARIES OF THE STUDIES
Study methodology
The first step, as a prerequisite for further studies, was the safety of the active ingredient for oral administration, which was demonstrated in studies complying with both OECD drug standards and EN ISO standards.
Already at a very early stage, preclinical studies and clinical observations demonstrated outstanding positive efficacy on oxidative stress, significant lactate reduction during physical exertion and improved well-being in combination with drug therapies. In addition, exceptional efficacy on various intestinal disorders has already been demonstrated at this early stage of application. These effects were accompanied by selective binding and elimination of toxins and ammonium in the gastrointestinal tract.
Scientific cooperation and publications
Quality and Homogeneity
Unique patented PMA technology optimizes effectiveness.
Allremedy medical devices are class IIb certified medical devices recognizable by the CE mark on the product in accordance with Directive 93/42 EEC.
As a medical device of class IIb, the safe continuous use of the PMA zeolite products is appropriately researched and proven.
For a Critical Review about zeolite see bibliography:
Critical Review on Zeolite Clinoptilolite Safety and Medical Applications in vivo
UPDATE OF OUR ONGOING RESEARCH COMPLETED STUDIES (2014 - 2022):
- Toxicological safety (OECD & ISO standards)
- Leaky gut (Lamprecht et al., 2015)
- NIS Reduction of liver values (Oberwinkler et al. 2014, unpublished)
- Osteoporosis (Pavelic Kraljevic et al., 2020)
- Better tolerability of chemotherapy (Vitale et al., 2020)
- Irritable bowel syndrome (IBS) (Petkov et al., 2021)
- Safety Study: Concentrations of Minerals & Pollutants (Pavelic Kraljevic et al., 2022)
- Osteoporosis: long-term study of fracture risk (Pavelic Kraljevic et al., 2022)
Study in publication / implementation / planning
- NIS RDS (Vogelsang et al., in finalisation)
- Oncology: Efficacy review by OS & PFS (Vitale et al., in publication).
- Microbiome (oral cavity): Periodontal disease and lichen ruber planus
Study design - focus: intestinal-liver axis
- Gut-liver axis: Alcohol and medicines
- Gut-liver axis incl. microbiome & diarrhoea: expansion of irritable bowel clientele
IN OUR RESEARCH WE CONCENTRATE ON THE INTESTINAL HEALTH, PARTICULARLY THE PROTECTIVE FUNCTION OF THE INTESTINAL WALL.
The loss of natural and protective/filtering function in the intestinal wall leads to the phenomenon known as the "leaky gut". Consequently, more unwelcome substances (e.g. bacterial components) find their way to the body from the intestine. This in turn not only jeopardize the intestine itself (local inflammatory reactions) but also affect the immune system and downstream organs (systemic inflammatory reactions).
In this context one speaks of a so-called endotoxemia. Because the impact on the organs can originate in the intestine about which science is increasingly concerned with the so-called intestine-body axes.
In the course of its research activities, we are also concerned with the effect of PMA zeolite on the intestine or the integrity of the intestinal wall (leaky gut) and its effects on our organism and the associated diseases.
Research results confirm the natural support of intestinal health in the following areas:
- For leaky gut and associated factors such as irritable bowel syndrome or elevated liver enzymes
- For better tolerability of medication therapies
- To support bone metabolism (osteoporosis)
- Pollutant prevention
- To support physical performance/strength through a strong intestine

Bibliography:
- Weber D., (2012): Study on the use of zeolite in the adjuvant treatment of eating disorders especially with regard to BMI and liver and kidney parameters
- Oberwinkler (2013): Influence of activated (modified) natural zeolite clinoptilolite to relieve the liver
- Lamprecht et al., (2015): Effects of zeolite supplementation on parameters of intestinal barrier integrity, inflammation, redoxbiology and performance in aerobically trained subjects
- Pavelić, Pavelić Kraljevic and Simović (2016): Effect of PMA-zeolite-clinoptilolite on the mineral metabolism and selected blood parameters
- Pavelić, Kraljevic and Pavelić, (2017): Effect of a PMA-zeolite-clinoptilolite on selected contaminants (heavy metals) after long term supplementation – safety evaluation
- Vitale et al., (2020): ZeOxaNMulti Trial: A Randomized, Double-Blinded, Placebo-Controlled Trial of Oral PMA-zeolite to prevent Chemotherapy-Induced Side Effects, in particular, Peripheral Neuropathy
- Böhm et al., (2020): Progress in health management through innovative diagnostics: The interpretation of ellipsoid erythrocytes under the dark field microscope in connection with Leaky Gut and the influence of PMA zeolite on the blood environment and the intestinal barrier
- Petkov, V. et al., (2021): PMA-zeolite can ameliorate inflammation in patients with irritable bowel syndrome – a randomized, double blinded, controlled pilot trial Running head: PMA-zeolite and IBS (Irritable Bowel Syndrome)
- Thoma, W. (2006): Clinical observation of the use of activated zeolite (= PMA zeolite) since April 2000.
- Knapitsch, Schmölzer, (2004): Randomized double-blind placebo-controlled study with Panaceo Sport on lactate levels and performance improvement
- Thoma, Gunzer, (2006) Clinical observation: The antioxidant effect of active volcanic mineral zeolite
(PMA zeolite) on the oxidative system - Abuja, P.M. (2006): An investigation of the antioxidant activities of PANACEO
- Schulz, N., (2007): Practical report concerning the use of the preparation Panaceo MED in the context of irritable bowel syndrome since 2004
- Bachl, N., (2011) Evaluation of the results of a randomised, double-blind study with Panaceo Sport to assess the effect on physical performance parameters
- Thoma, (2011): Clinical Observation of Administration of Panaceo-med (Representative Sample of Patients)
- Triebnig, (2011): Activated natural zeolite clinoptilolite in complementary medicine. Proven roborans in severe diseases, recommended adjuvant in chemo- and radiotherapy. (Note: Evaluation of 150 representative patient records from a long-term application observation on over 2,000 patients in the form of documented medical cases over the period of 10 years using PMA zeolite)
